Contact
Dr Mélanie Leroy, project manager
Pr Frédéric Gottrand, scientific coordinator
Long term outcome of esophageal atresia: transomics profiles in adolescence

Esophageal atresia (EA), a malformation of the esophagus present at birth, is characterized by the interruption of the continuity of the esophagus, which then terminates in a blind pouch. Food or saliva cannot be carried into the stomach. It affects an average of 150 births per year in France, making it a rare disease.
Surgery is necessary to reconnect the esophagus. Although this intervention allows the vast majority of children to survive the neonatal period, health problems such as gastroesophageal reflux, difficulty eating, respiratory issues, as well as growth problems may persist throughout life
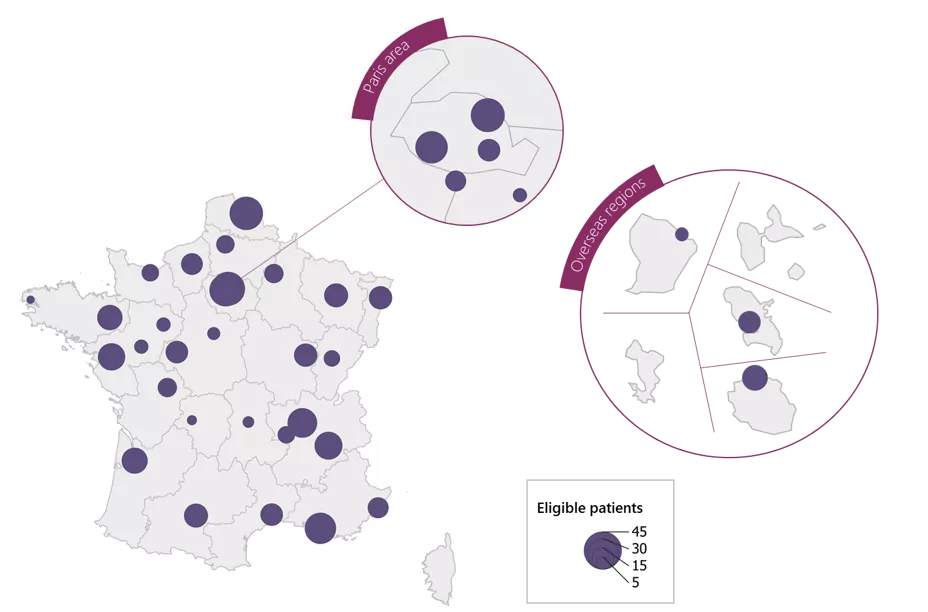
The objective of the project is to create a prospective cohort of adolescents aged 13/14 years, nested within the national registry of esophageal atresia (EA). In addition to clinical data, a biobank of esophageal mucosa and plasma samples will be established.
Once the clinical data has been collected and omics data (derived from the analysis of biological samples from the biobank) generated, they will be analyzed by project partners to assess the long-term outcomes of EA and to establish multi-omics profiles.

November 04th 2025
It is composed of a cross-functional team responsible for monitoring and ensuring the smooth progress of the project. It consists of:
It exercises an advisory role on academic strategy, in connection with its international scientific expertise. It consists of:
Recruited from within the AFAO (Association Française de l'Atrésie de l'Œsophage), these are parents of children born with EA, who are willing volunteers actively participating in various stages of the project's development and implementation.
X
X
The raw data is not yet accessible but will be made available on the France Cohortes platform
Dr Mélanie Leroy, project manager
Pr Frédéric Gottrand, scientific coordinator
This work received support from the Agence Nationale de la Recherche as part of France 2030 program under reference ANR-21-PMRB-0011, as well as funding from the IRCEM Foundation.
